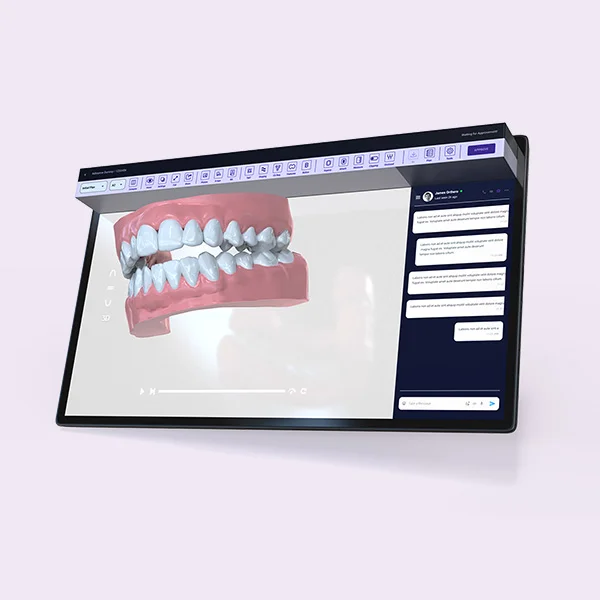
In healthcare, usability is safety. Products must remain reliable across complex workflows, repeated cleaning cycles, and strict stakeholder expectations. We support MedTech and healthcare teams by combining human-centered design thinking with production-aware execution, focusing on clarity, risk reduction, and supplier-ready delivery.
Regulated Product Development
(MedTech & Healthcare)
Human-centered product delivery for high-stakes environments.
- Human-centered design for clinical workflows and constraints
- Production-aware delivery and structured documentation
- Clarity-driven handoff for engineering and suppliers
What MedTech delivery typically includes
Healthcare products require clarity, reliability, and context-driven usability. We align execution across stakeholders and support delivery logic that reduces risk in downstream handoff.
- Workflow-aware design direction (clinical context)
- Ergonomics, clarity, and error prevention principles
- CMF choices suitable for hygiene and durability
- Robust documentation for supplier and engineering alignment
- Prototype learnings and iteration decision traceability
What you receive
- Context definition: users, workflows, constraints
- Design intent pack optimized for clarity and usability
- Engineering integration and manufacturing assumptions
- Prototype/validation notes and iteration rationale
- Handoff-ready documentation aligned with execution needs
- Supplier-ready outputs (as agreed)
Designed to reduce risk
MedTech teams must align multiple stakeholders. Our structured workflow supports better governance and traceable decisions, while our delivery focuses on usability, clarity, and production readiness.
Key outcomes:
- Reduced ambiguity during engineering and supplier handoff
- Faster alignment through clear milestones and decisions
- Stronger usability quality in real-world workflows
Delivered via Designlogger
Controlled assets, structured folders, traceable approvals.
MedTech programs demand controlled documentation and traceable decisions. We deliver through Designlogger as the single source of truth for files, revisions, approvals, and handoff packages.
Need MedTech delivery with clarity and production awareness?
Book a 15-minute introduction call to discuss scope, stakeholders, and timelines.